In this article, we will discuss and answer all the questions related to the reaction of copper oxide and sulphuric acid in detail. But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. Sulphuric acid is a strong acid that is formed by oxidizing solutions of sulphur dioxide. The sulfuric acid formula is
Reacting Copper Oxide with Sulphuric Acid
Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt . Copper (II) oxide, is a black solid, which, when reacted with sulphuric acid creates a cyan-blue coloured chemical called copper II sulfate. Copper (II) oxide reacts with sulfuric acid to create water and copper (II) sulfate. This reaction could be classified as a double displacement reaction or a neutralization reaction. Copper sulphate takes on a bright blue colour
Writing the Equation form of Copper oxide + Sulphuric Acid
This chemical reaction can be written as the following:
Copper oxide(solid) + Sulphuric Acid (aqueous)-> Copper Sulphate (aqueous)+ Water(liquid) To find out how you can make Copper Sulphate at home check out this article.
What happens when the copper reacts with concentrated Sulphuric acid? The reduction potential of diluted sulphuric acid is higher than that of hydrogen. Copper is unable to displace hydrogen from non-oxidizing acids, for instance, hydrochloric acid or diluted sulphuric acid. In other words, we can say that the copper does not react with the diluted sulphuric acid. However, it does react with the concentrated sulphuric acid because sulphuric acid in concentrated form is an oxidizing agent. When copper gets heated with concentrated sulphuric acid, there is a redox reaction and the acid turns into sulfur dioxide. The equation of this chemical reaction is given below:
What is the balanced equation for copper oxide and Sulphuric acid? The copper oxide and sulphuric acid balanced equation is given below:
Why do copper oxide and Sulphuric acid turn blue? We all know that the copper oxide + sulfuric acid reaction results in a blue-colored chemical. But have you ever wondered why copper oxide sulphuric acid reaction results in a blue-colored chemical? Well, we will answer this question in detail here. Copper oxide is a black-colored solid. When it reacts with sulphuric acid, it produces a cyan-blue colored chemical which is known as copper sulphate. The blue color is due to the formation of soluble salt. The copper and sulphate ions dissociate as the copper sulphate gets dissolved in water. Although there is no change in the effect, however, the nature of the split between t2g and eg orbitals in this new complex is such that it absorbs reddish-orange light. Due to this absorption, you will see a bluish-colored solution.
Does sulfuric acid dissolve copper? No, sulphuric acid cannot dissolve the copper. However, if dissolution is observed, it can be due to one of the following two reasons:
There is a possibility that the surface of copper metal powder is partially oxidized into
The formation of a vortex during the agitation. A tiny amount of air (oxygen) that was introduced to a leach solution acted like an oxidant.
What salt is produced when copper oxide reacts with hydrochloric acid? The reaction of copper and hydrochloric acid is not possible. However, copper oxide can react with this acid. When a metal reacts with an acid, a redox reaction occurs. Because of the higher reduction potential of copper as compared to hydrogen, it is unable to react with non-oxidizing acids like sulphuric acid and hydrochloric acid.
But copper oxide is not a metal, rather it is a metal oxide. Metal oxides are basic substances that can react with acids to form salt and water. These acid-base reactions are also known as neutralization and are non-redox in nature.
Being a weak base, copper oxide reacts with HCL easily to generate a soluble copper chloride and water. The equation of this chemical reaction is given below:


 .
. 

 reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate
reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate  is generated. The equation of this reaction is given below:
is generated. The equation of this reaction is given below: 


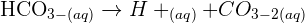

 . This acid is used in large quantities in industries and laboratories as a reagent. The concentrated form of sulphuric acid is a dense, oily, and corrosive. The other compound copper oxide is a compound that is formed when two elements copper and oxygen react with each other. Its formula is CuO.
. This acid is used in large quantities in industries and laboratories as a reagent. The concentrated form of sulphuric acid is a dense, oily, and corrosive. The other compound copper oxide is a compound that is formed when two elements copper and oxygen react with each other. Its formula is CuO. 



 . Sulphuric acid can dissolve the copper surface that has been oxidized
. Sulphuric acid can dissolve the copper surface that has been oxidized