The reaction between copper carbonate and sulphuric acid will be a neutralization reaction. Copper carbonate will acts as a base, however, sulphuric acid is an acid. This reaction can also be viewed as a double displacement reaction too. Let's understand the nature of this reaction and what will be the final product of this reaction. 
Neutralization Reaction
The word neutralization means that the PH of the product will be around  . Sometimes it can be fix
. Sometimes it can be fix  , sometimes it is around
, sometimes it is around  . We consider from
. We consider from  to
to 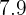 as neutrals. PH stands for Potential of Hydrogen. Hydrogen is an acidic element and if it is combined with any element or compound, it will result in an acidic compound. The PH scale measures the power of hydrogen in a compound.
as neutrals. PH stands for Potential of Hydrogen. Hydrogen is an acidic element and if it is combined with any element or compound, it will result in an acidic compound. The PH scale measures the power of hydrogen in a compound.
Understanding PH Scale
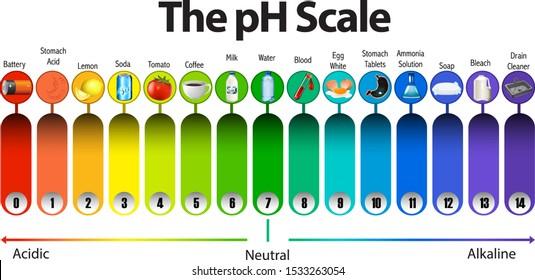 The above picture represents the pH scale starting from 1 and ending on 14. There are three regions in the pH scale. From pH 1 to pH 6, it is the acidic region where 1 being the most acidic region and 6 being the weakest acidic region. From pH 8 to pH 14, it is the alkaline region where 8 being the weakest alkaline and 14 being the most alkaline region. Their color represents their value. The basic test for acid and alkaline is through litmus paper. It is a special type of paper that responds to acidic or basic mediums. Litmus paper comes in two colors, red and blue. If the blue litmus paper turns red, that means your substance is acidic and if remains blue, you are in an alkaline medium. In the case of red litmus paper, if it turns blue that means the medium is acidic, however, if it remains blue that indicates the medium is alkaline.
The above picture represents the pH scale starting from 1 and ending on 14. There are three regions in the pH scale. From pH 1 to pH 6, it is the acidic region where 1 being the most acidic region and 6 being the weakest acidic region. From pH 8 to pH 14, it is the alkaline region where 8 being the weakest alkaline and 14 being the most alkaline region. Their color represents their value. The basic test for acid and alkaline is through litmus paper. It is a special type of paper that responds to acidic or basic mediums. Litmus paper comes in two colors, red and blue. If the blue litmus paper turns red, that means your substance is acidic and if remains blue, you are in an alkaline medium. In the case of red litmus paper, if it turns blue that means the medium is acidic, however, if it remains blue that indicates the medium is alkaline.
Neutralization Reaction Results
Neutralization reaction occurs when one compound is acidic and another compound is basic. If both compounds are acidic, there will be no reaction. The same goes for both basic compounds reacting to each other. Neutralization reaction usually results in salt and water, we will explain why water is emitted from neutralization reaction. However, if you check closely, copper is in carbonate form. This means copper has carbon dioxide with it. Whenever carbonates react with acid, they will also release carbon dioxide with water and salt.
Observation of Copper Carbonate and Sulphuric Acid Reaction
Copper Carbonate
Copper carbonate is a compound that contains a transition element (which is copper) and a carbonate compound. Since copper is a transition element, it will show you color, which is why the color of the solid is green. The molar mass of this compound is 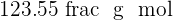 and is insoluble in water. The ion of copper is
and is insoluble in water. The ion of copper is 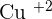 and the ion of carbonate is
and the ion of carbonate is 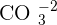 . They both go under an ionic reaction and that will result in copper carbonate
. They both go under an ionic reaction and that will result in copper carbonate 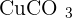 . Because of the carbonate properties, copper carbonate is
. Because of the carbonate properties, copper carbonate is  . It means it is an alkaline compound.
. It means it is an alkaline compound.
Sulphuric Acid
Sulphuric acid is also a compound that contains hydrogen, sulfur, and oxygen elements. Sulphuric acid is known as the king of acids because of its highly acidic property. It is a very strong acid having pH of 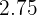 . It is usually in aqueous form and is a colorless solution. The molar mass of this compound is
. It is usually in aqueous form and is a colorless solution. The molar mass of this compound is 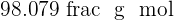 . Sulphuric acid is made industrially where sulfur and oxygen go under various steps to form sulphuric acid. The name of this process is Contact Process.
. Sulphuric acid is made industrially where sulfur and oxygen go under various steps to form sulphuric acid. The name of this process is Contact Process.
Observations
Acid-base reactions are highly exothermic. It means these reactions will release energy in terms of heat. However, the amount of energy-releasing varies from reaction to reaction. One thing is for sure, you will observe a colorless gas been emitted. That gas is carbon dioxide. The reaction kinetics in an account then increasing the temperature and increasing the sulphuric acid concentration will enhance the rate of reaction. If this reaction is done at RTP conditions, it will take 10 - 20 seconds for a complete neutralization reaction. Once the reaction is finished, the resultant product color will be blue.
Copper Carbonate and Sulphuric Acid Balanced Equation
Here is the moment you all have been waiting for, if copper carbonate reacts with sulphuric acid, it will result in copper(II) sulfate, water, and carbon dioxide. Below is the reaction:  There is no need to balance the equation because it is already balanced. Let's break this equation and check its ionic equation:
There is no need to balance the equation because it is already balanced. Let's break this equation and check its ionic equation: 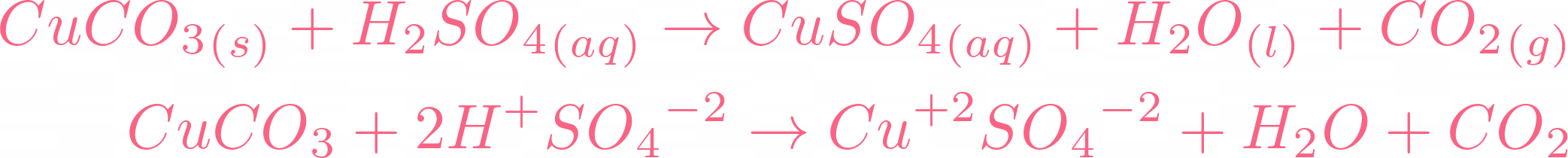 All the compounds are broken in their ions. In the above equation,
All the compounds are broken in their ions. In the above equation,  is a spectator ion. Spectator ions don't involve in the ionic reaction. Now we will cut off the spectator ion that is common on both sides of the equation and add their state.
is a spectator ion. Spectator ions don't involve in the ionic reaction. Now we will cut off the spectator ion that is common on both sides of the equation and add their state. 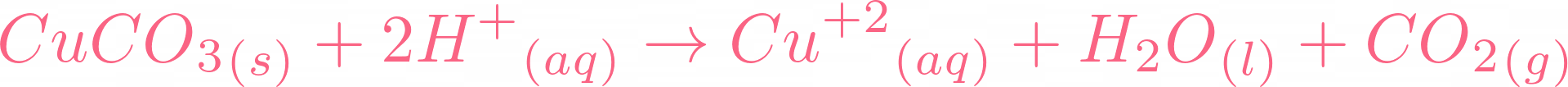 The above ions are taking part in the reaction. You can check out that water and carbon dioxide are being produced. If you check all ionic equations of acid-base reactions, you will find that water is always produced. In the case of carbonates, carbon dioxide and water are produced.
The above ions are taking part in the reaction. You can check out that water and carbon dioxide are being produced. If you check all ionic equations of acid-base reactions, you will find that water is always produced. In the case of carbonates, carbon dioxide and water are produced.
Double Displacement Reaction
Any reaction that exchanges anions or cations are known as displacement reaction. There are two types of displacement reactions, single displacement reaction, and double displacement reaction. If two reactants exchanges cations and anions to make new products, that reaction is called a double displacement reaction. On the other hand, if one reactant exchanges cation or anion from the other reactant, we call it a single displacement reaction. Acid-base reactions are always double displacement reactions. 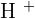 and
and  are replaced to form water and the rest ions react with each other to form salt. Replacement occurs on the basis of reactivity series and that is a different domain, let's stick to acid-base for now.
are replaced to form water and the rest ions react with each other to form salt. Replacement occurs on the basis of reactivity series and that is a different domain, let's stick to acid-base for now.
Reaction Between Hydrochloric Acid and Copper Carbonate
At this point, you might be thinking what happens if we react hydrochloric acid with copper carbonate? Let's get to the result, the equation will be something like this:

As the name tells you hydrochloric acid is another type of acid. If we compare hydrochloric acid with sulphuric acid then hydrochloric acid is weaker than sulphuric acid. Its pH value is 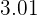 but it is considered a strong acid. The power of acids is determined by their dissociation in water and hydrochloric acid dissociates completely in water. Of course, changing of acid will result in a new equation but there are some things that will remain common, and guess what they are? If you guessed carbon dioxide and water then you are 100% correct! If acid reacts with a base, it will always release water, but if the base is carbonate, it will release carbon dioxide and water. The only change we will see is in salt.
but it is considered a strong acid. The power of acids is determined by their dissociation in water and hydrochloric acid dissociates completely in water. Of course, changing of acid will result in a new equation but there are some things that will remain common, and guess what they are? If you guessed carbon dioxide and water then you are 100% correct! If acid reacts with a base, it will always release water, but if the base is carbonate, it will release carbon dioxide and water. The only change we will see is in salt.  The salt produced in this reaction is copper II chloride. The reaction requires 2 moles of hydrochloric acid to completely react with 1 mole of copper carbonate. The color of copper chloride is blue-green and yes, this reaction is a double displacement reaction. Let's check its ionic equation:
The salt produced in this reaction is copper II chloride. The reaction requires 2 moles of hydrochloric acid to completely react with 1 mole of copper carbonate. The color of copper chloride is blue-green and yes, this reaction is a double displacement reaction. Let's check its ionic equation:
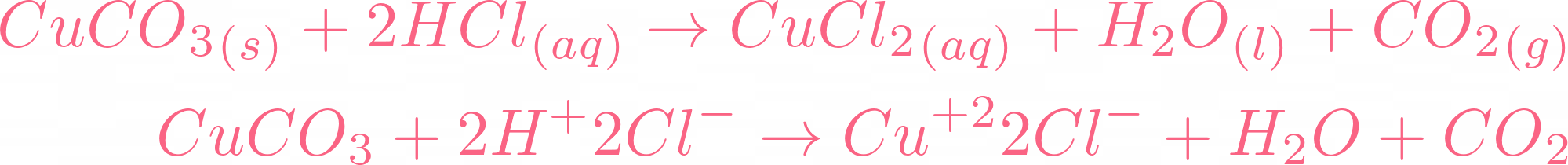
Now we will cut off all the spectator ions. In the above ionic equation, the spectator ion is 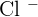 , after cutting off chlorine, this will be the final ionic equation with their states:
, after cutting off chlorine, this will be the final ionic equation with their states: 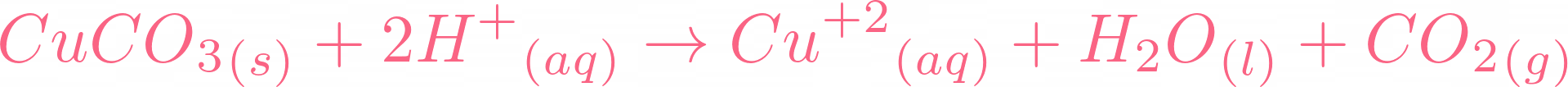
Does Sulphuric Acid React with Copper?
There are two stories on this topic. Usually, we use diluted solutions of acids and bases for reaction. You must be thinking why? This is to lower the amount of concentration of both, acid and alkali. This also results in decrease in pH values because 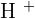 and
and  are involved with water molecule. This is necessary for laboratory experiments because concentrated bases and acids are very dangerous to handle. A small mistake can result in a big disaster! Furthermore, diluting these chemicals does lower the concentration of
are involved with water molecule. This is necessary for laboratory experiments because concentrated bases and acids are very dangerous to handle. A small mistake can result in a big disaster! Furthermore, diluting these chemicals does lower the concentration of 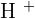 and
and  ions but it is good for you because it will be easier to reach the pH
ions but it is good for you because it will be easier to reach the pH  than using concentrated solutions. In addition, a tremendous amount of energy will be released in the reaction of concentrated acids and bases that can trigger many alarms in a laboratory. The first rule of performing an experiment is to be safe, don't forget, safety is first! If you add copper metal to diluted sulphuric acid, there will be no reaction. Here is the reason, remember about the displacement reaction? Displacement reaction occurs on the reactivity series. The reactivity series is a series of elements that are listed according to their reactivity. The top on the list is the most reactive and the bottom on the list is the least reactive. If you check the reactivity series, copper is below hydrogen which shows copper is less reactive than hydrogen. Displacement reaction occurs when a most reactive cation replaces the less reactive cation. This condition doesn't fulfill in the above case. However, if you tried to react copper with concentrated sulphuric acid, then there is a possible reaction. The reaction of concentrated sulphuric acid and copper is not an acid-base reaction, it is a redox reaction. Below is the chemical equation of this reaction:
than using concentrated solutions. In addition, a tremendous amount of energy will be released in the reaction of concentrated acids and bases that can trigger many alarms in a laboratory. The first rule of performing an experiment is to be safe, don't forget, safety is first! If you add copper metal to diluted sulphuric acid, there will be no reaction. Here is the reason, remember about the displacement reaction? Displacement reaction occurs on the reactivity series. The reactivity series is a series of elements that are listed according to their reactivity. The top on the list is the most reactive and the bottom on the list is the least reactive. If you check the reactivity series, copper is below hydrogen which shows copper is less reactive than hydrogen. Displacement reaction occurs when a most reactive cation replaces the less reactive cation. This condition doesn't fulfill in the above case. However, if you tried to react copper with concentrated sulphuric acid, then there is a possible reaction. The reaction of concentrated sulphuric acid and copper is not an acid-base reaction, it is a redox reaction. Below is the chemical equation of this reaction:  Copper reacts with 2 moles of sulphuric acid (concentrated) to produce copper sulfate, water, and sulfur dioxide. Let's study its redox, concentrated sulphuric acid is a reducing agent. That means
Copper reacts with 2 moles of sulphuric acid (concentrated) to produce copper sulfate, water, and sulfur dioxide. Let's study its redox, concentrated sulphuric acid is a reducing agent. That means  is converted to
is converted to 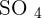 to oxidize copper from
to oxidize copper from 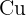 to
to 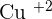 . This reaction requires external heat to reach its activation energy.
. This reaction requires external heat to reach its activation energy.



 . Sometimes it can be fix
. Sometimes it can be fix 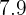 as neutrals. PH stands for Potential of Hydrogen. Hydrogen is an acidic element and if it is combined with any element or compound, it will result in an acidic compound. The PH scale measures the power of hydrogen in a compound.
as neutrals. PH stands for Potential of Hydrogen. Hydrogen is an acidic element and if it is combined with any element or compound, it will result in an acidic compound. The PH scale measures the power of hydrogen in a compound.  The above picture represents the pH scale starting from 1 and ending on 14. There are three regions in the pH scale. From pH 1 to pH 6, it is the acidic region where 1 being the most acidic region and 6 being the weakest acidic region. From pH 8 to pH 14, it is the alkaline region where 8 being the weakest alkaline and 14 being the most alkaline region. Their color represents their value. The basic test for acid and alkaline is through litmus paper. It is a special type of paper that responds to acidic or basic mediums. Litmus paper comes in two colors,
The above picture represents the pH scale starting from 1 and ending on 14. There are three regions in the pH scale. From pH 1 to pH 6, it is the acidic region where 1 being the most acidic region and 6 being the weakest acidic region. From pH 8 to pH 14, it is the alkaline region where 8 being the weakest alkaline and 14 being the most alkaline region. Their color represents their value. The basic test for acid and alkaline is through litmus paper. It is a special type of paper that responds to acidic or basic mediums. Litmus paper comes in two colors, 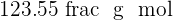 and is insoluble in water. The ion of copper is
and is insoluble in water. The ion of copper is 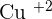 and the ion of carbonate is
and the ion of carbonate is 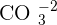 . They both go under an ionic reaction and that will result in copper carbonate
. They both go under an ionic reaction and that will result in copper carbonate 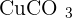 . Because of the carbonate properties, copper carbonate is
. Because of the carbonate properties, copper carbonate is  . It means it is an alkaline compound.
. It means it is an alkaline compound. 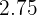 . It is usually in aqueous form and is a colorless solution. The molar mass of this compound is
. It is usually in aqueous form and is a colorless solution. The molar mass of this compound is 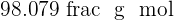 . Sulphuric acid is made industrially where sulfur and oxygen go under various steps to form sulphuric acid. The name of this process is Contact Process.
. Sulphuric acid is made industrially where sulfur and oxygen go under various steps to form sulphuric acid. The name of this process is Contact Process.  There is no need to balance the equation because it is already balanced. Let's break this equation and check its ionic equation:
There is no need to balance the equation because it is already balanced. Let's break this equation and check its ionic equation: 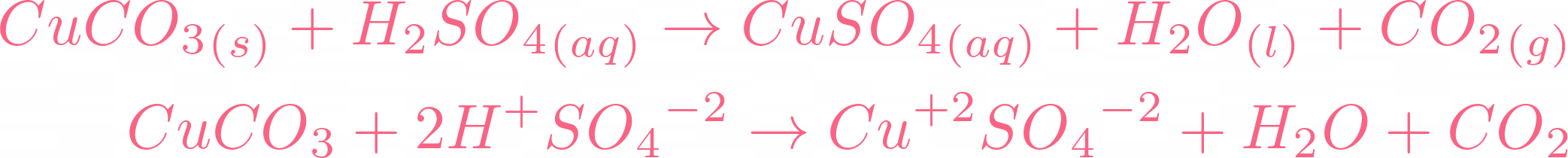 All the compounds are broken in their ions. In the above equation,
All the compounds are broken in their ions. In the above equation,  is a spectator ion. Spectator ions don't involve in the ionic reaction. Now we will cut off the spectator ion that is common on both sides of the equation and add their state.
is a spectator ion. Spectator ions don't involve in the ionic reaction. Now we will cut off the spectator ion that is common on both sides of the equation and add their state. 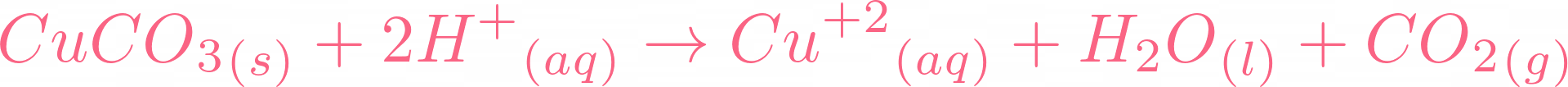 The above ions are taking part in the reaction. You can check out that water and carbon dioxide are being produced. If you check all ionic equations of acid-base reactions, you will find that water is always produced. In the case of carbonates, carbon dioxide and water are produced.
The above ions are taking part in the reaction. You can check out that water and carbon dioxide are being produced. If you check all ionic equations of acid-base reactions, you will find that water is always produced. In the case of carbonates, carbon dioxide and water are produced. 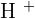 and
and  are replaced to form water and the rest ions react with each other to form salt. Replacement occurs on the basis of reactivity series and that is a different domain, let's stick to acid-base for now.
are replaced to form water and the rest ions react with each other to form salt. Replacement occurs on the basis of reactivity series and that is a different domain, let's stick to acid-base for now. 
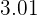 but it is considered a strong acid. The power of acids is determined by their dissociation in water and hydrochloric acid dissociates completely in water. Of course, changing of acid will result in a new equation but there are some things that will remain common, and guess what they are? If you guessed carbon dioxide and water then you are 100% correct! If acid reacts with a base, it will always release water, but if the base is carbonate, it will release carbon dioxide and water. The only change we will see is in salt.
but it is considered a strong acid. The power of acids is determined by their dissociation in water and hydrochloric acid dissociates completely in water. Of course, changing of acid will result in a new equation but there are some things that will remain common, and guess what they are? If you guessed carbon dioxide and water then you are 100% correct! If acid reacts with a base, it will always release water, but if the base is carbonate, it will release carbon dioxide and water. The only change we will see is in salt.  The salt produced in this reaction is copper II chloride. The reaction requires 2 moles of hydrochloric acid to completely react with 1 mole of copper carbonate. The color of copper chloride is
The salt produced in this reaction is copper II chloride. The reaction requires 2 moles of hydrochloric acid to completely react with 1 mole of copper carbonate. The color of copper chloride is 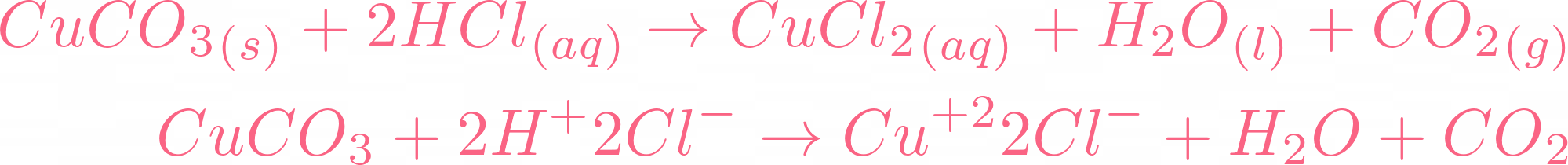
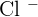 , after cutting off chlorine, this will be the final ionic equation with their states:
, after cutting off chlorine, this will be the final ionic equation with their states: 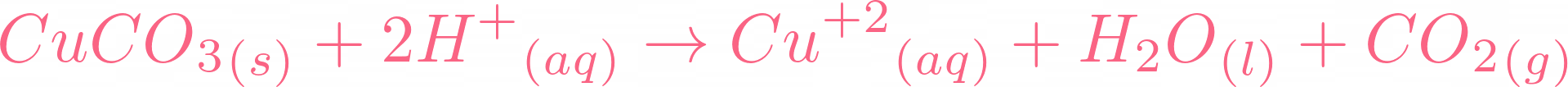
 Copper reacts with 2 moles of sulphuric acid (concentrated) to produce copper sulfate, water, and sulfur dioxide. Let's study its redox, concentrated sulphuric acid is a reducing agent. That means
Copper reacts with 2 moles of sulphuric acid (concentrated) to produce copper sulfate, water, and sulfur dioxide. Let's study its redox, concentrated sulphuric acid is a reducing agent. That means  is converted to
is converted to 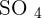 to oxidize copper from
to oxidize copper from 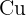 to
to